The long-term goal of the Hanlon Lab is to advance the field of chromosome biology by utilizing the B chromosomes as tools to expand our knowledge of chromosome dynamics, behavior, and evolution.
The propagation and maintenance of the B chromosomes in their original stock is due to female meiotic drive
In several other species, B chromosomes have evolved specialized drive systems that allow them to be passed from parent to progeny in a biased, Mendelian-law-breaking fashion. The D. melanogaster B chromosomes do not have their own drive system but somehow have been maintained for many generations. Our work shows that having only one functional copy of matrimony can promote the biased transmission of B chromosomes in a sex-specific manner, indicating that one role of matrimony may be to work as a suppressor of drive during female meiosis. Investigating the mechanism of this female meiotic drive will be through two complementary approaches: a molecular approach that will examine which domains of Mtrm are critical for its suppression of meiotic drive, and a cellular approach that will enable the visualization of B chromosomes dynamics during a live meiotic division. Together, these molecular and cellular approaches will allow the larger picture of the mechanism behind female meiotic drive of the B chromosomes to emerge.
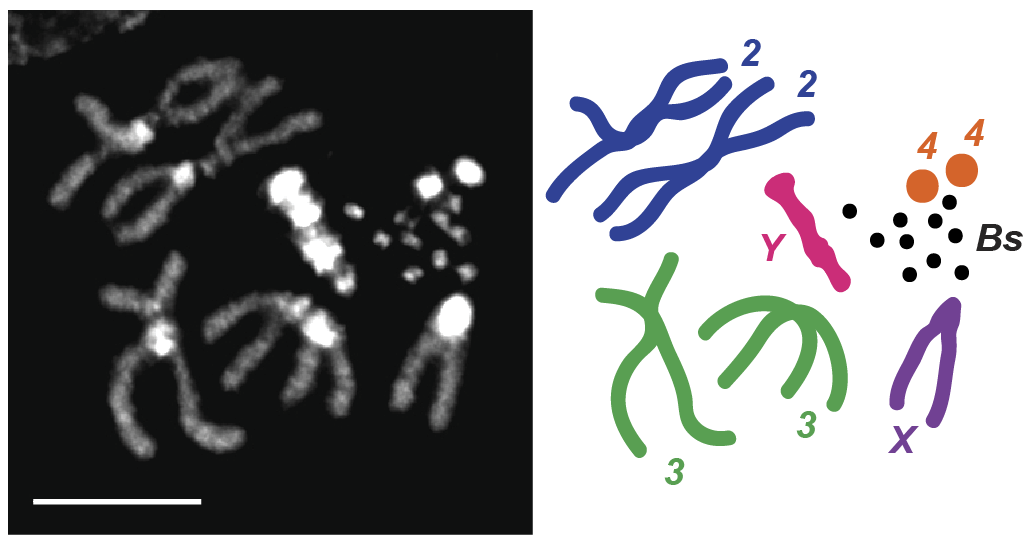
Karyotype from a male carrying 10 B chromosomes (left) and itsillustrated representation (right). The original B chromosome stock averages 10-12 B chromosomes per fly. Adapted from Hanlon et al., 2018. Scale bar: 5 µm.
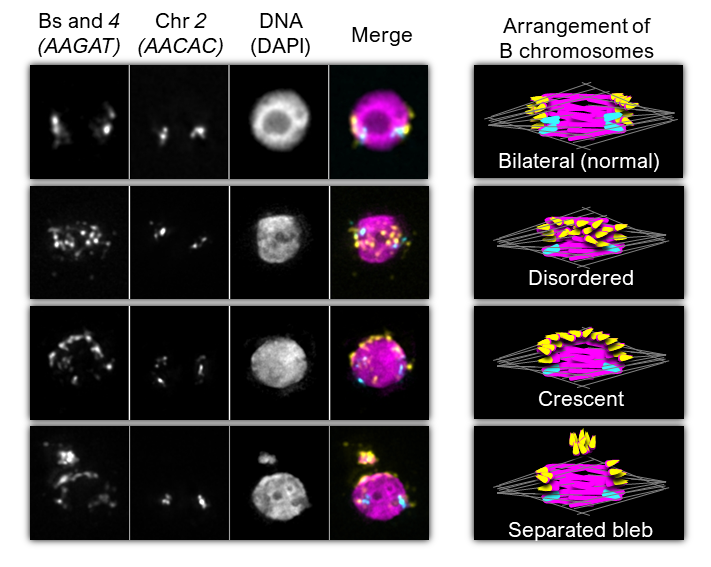
The B chromosomes display aberrant behavior at the first metaphase arrest during female meiosis in genetic backgrounds that promote meiotic drive
Before the first meiotic division in females, the oocyte is arrested in metaphase and the chromosomes congress into a single DNA mass. The location of the chromosomes within this mass is highly correlative with the pole to which they will eventually segregate, indicating the proper positioning of chromosomes at the metaphase arrest is critical for their proper segregation. Our preliminary work indicates that the B chromosomes behave as expected going into the metaphase arrest in wild-type females, however in females that display high levels of meiotic drive the B chromosomes display aberrant behavior and are improperly positioned within the oocyte. Current work in the lab is focusing on the interplay between the irregular arrangement of the B chromosomes and their biased segregation during female meiosis.
B chromosomes are an emerging model system for understanding how small supernumerary chromosomes can be detrimental to human health
While the gain of a whole chromosome is generally lethal, the gain of part of a chromosome (i.e., segmental aneuploidy) can be viable. Small supernumerary marker chromosomes (sSMCs) are structurally abnormal chromosomes that are additions to the standard chromosome complement and are usually smaller than any of the essential chromosomes. Present in ~1/2300 births, sSMCs have been linked to specific syndromes (e.g., Emanuel and Pallister-Killian) and can be a potent cause of infertility, fetal loss, and intellectual and developmental disabilities. Like sSMCs, the B chromosome is carried in addition to the standard chromosome complement and was derived from one of the essential chromosomes. The similarities between sSMCs and B chromosomes combined with the power of D. melanogaster as a model for human meiosis makes the D. melanogaster B chromosome system well equipped to become the ideal genetic system for the study of supernumerary chromosomes during meiosis.
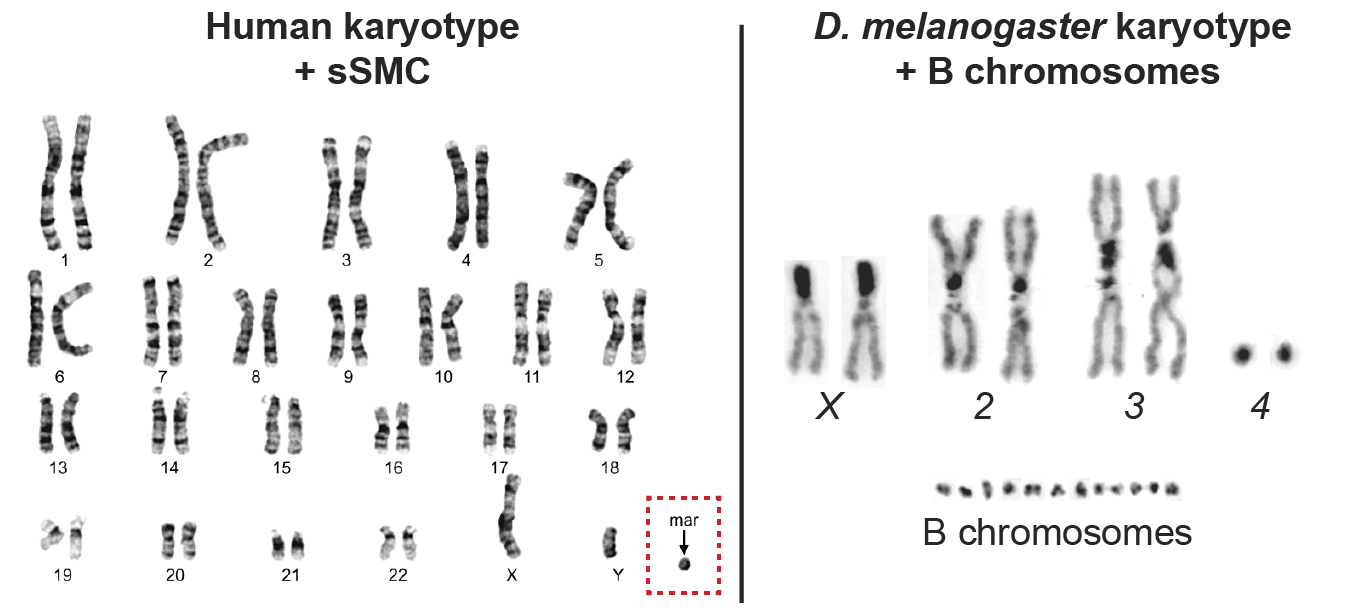
Comparison of a human metaphase chromosome spread from an infertile male carrying a single sSMC (in dashed-line box) to a D. melanogaster chromosome spread carrying 12 supernumerary B chromosomes. Human karyotype from Song, S. H. et al. World J MensHealth 35 (2017).